Test-specific transport instructions are listed on the Lab Test Menu.
All specimens shipped to WAPHL regardless of the tests ordered must be packaged according to IATA, OSHA, and DOT requirements.
Important: Biological substances, including specimens shipped for testing purposes, are regulated as a Hazardous Material by the US Department of Transportation (DOT). It is the shipper's responsibility to follow regulations for the shipment of Division 6.2 Infectious Substances when sending biological material, human or animal specimens, or cultures of microorganisms to the Washington State Public Health Laboratory (WAPHL). The DOT's Hazardous Material Regulations are contained in the Code of Federal Regulations section 49 Parts 171-180.
Shipping of diagnostic specimens and infectious materials must be performed or supervised by a person who has received training in the packaging and shipping of Division 6.2 Infectious Substances. Training is available for free online through the CDC.
Shipping Diagnostic Specimens
All diagnostic specimens, including blood and blood products, tissue and tissue fluids, excreta, and secreta, must be packaged using an IATA- and DOT-compliant triple packaging system that prevents leakage or damage.
- Primary containers must be watertight for liquid specimens. Individual containers must be wrapped or separated in a way that prevents potential damage. Absorbent material, preferably cellulose wadding, should be located between the primary and secondary containers. Urine containers are discouraged for the transport of liquid specimens; please remove liquid specimens to a labeled conical tube for transport. Screw-top, snap-on, and push-on lids should be sealed with paraffin laboratory tape such as ParafilmTM.
- Secondary container must be watertight, but is not required to be rigid. A sealed plastic bag is acceptable.
- Outer packaging must be rigid and constructed of sturdy fiberboard, plastic, or other solid material. Diagnostic specimens meeting DOT and IATA requirements as non-infectious substances should be marked “Exempt Human Specimen” or “Exempt Animal Specimen” in compliance with IATA specifications.
Dried blood spots and fecal occult blood screens are not subject to regulation as infectious substances and require no special markings for shipment. Use only designated absorbent paper specimen collection systems for the collection, storage, and transport of dried blood spots.
Important: Wet Ice is not permitted for transport of specimens to WAPHL.
Shipping Category A and B Specimens
Category A Shipping Guide (PDF)
Category B Shipping Guide (PDF)
Follow the most current IATA or DOT classification requirements for classifying specimens as Category A, B, or Exempt. Reference the following checklists for guidance in Category A and B packaging. These checklists are not intended to be a sole resource when preparing shipments and persons performing shipping and packaging of infectious substances must have received training appropriate to the level of the task. If elements in these checklists are missing for any package containing a Category A or B specimen, the package is improperly prepared according to WAPHL requirements and may be in violation of federal regulations. Packages that do not meet requirements may be rejected for testing.
Category A packaging and labelling (IATA Packing Instruction 620)
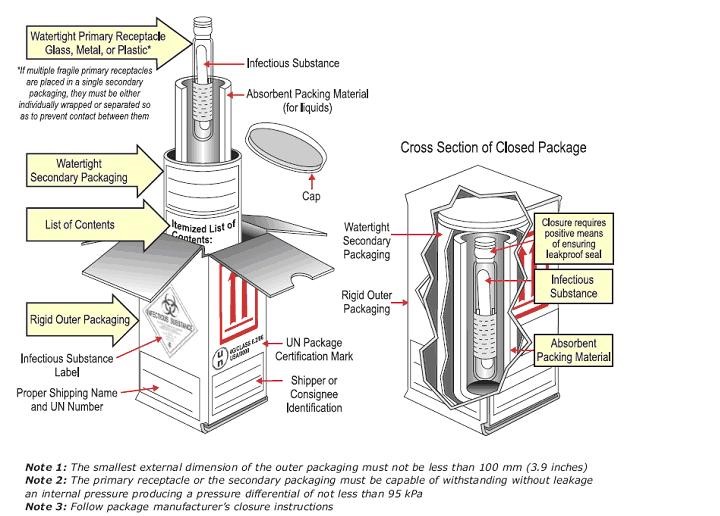
Image source: Transporting Infectious Substances Safely, Guide to Changes. US Department of Transportation Pipeline and Hazardous Materials Safety Administration
Category A Checklist
- Packaging and documents are prepared by a person certified in Packaging and Shipping Division 6.2 Infectious Substances
- If a manufactured Category A shipping system is used, the manufacturer's instructions have been followed and no parts have been incorporated from other Category A shipping systems
- Triple packaging system is employed with primary specimen container, secondary packaging, and outer packaging according to IATA and DOT requirements
- Primary container is sealed (with ParafilmTM or heat-shrink wrap, or other sealant) such that it is guaranteed not to leak and the cap cannot loosen
- Primary container is accompanied by cushioning material and cellulose absorbent
- Secondary packaging is pressure-resistant to 95kPa
- Contents are listed on requisition or specimen submission form or otherwise listed outside secondary packaging
- Paperwork is protected from condensation from chemical ice packs, if used
- Secondary packaging is secured inside outer packaging by extra cushioning material if necessary
- If dry ice is used, proper dry ice shipping protocols have been referenced and followed
- Outer package is UN certified Category A box
- Outer package labelled with class 6 hazard diamond, and proper shipping and technical names
- 24 hour emergency contact is required on the outer package. Emergency contact must be able to provide spill response instructions; delays in response time are finable by the USDOT
- Full name, phone number, and address of responsible person, shipper, and consignee are listed on outer package
- 3 signed copies of the red-striped shippers declaration of dangerous goods attached to package
Category B Packaging and Labelling (IATA Packing Instruction 650)
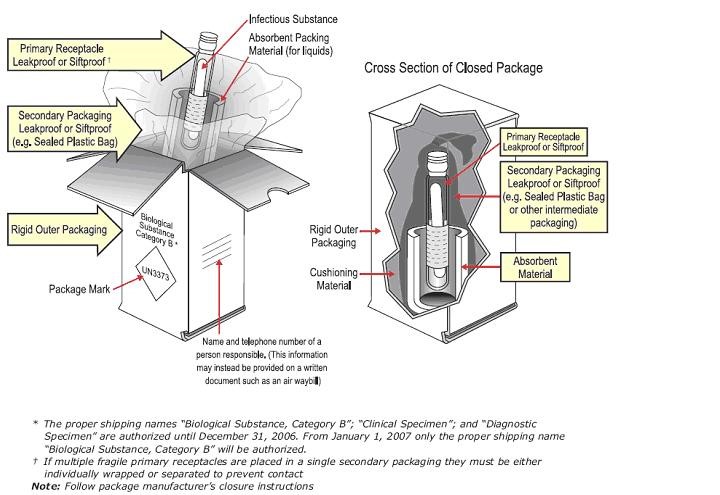
Image source: Transporting Infectious Substances Safely, Guide to Changes. US Department of Transportation Pipeline and Hazardous Materials Safety Administration
Category B Checklist
- Packaging and documents are prepared by a person with awareness of category B shipping requirements
- Triple packaging system is employed with primary specimen container, secondary packaging, and outer packaging according to IATA and DOT requirements
- Primary container sealed (with ParafilmTM or heat-shrink wrap, or other sealant) such that it is guaranteed not to leak and the cap cannot loosen
- Primary container is accompanied by cushioning material and cellulose absorbent
- Secondary container pressure-resistant to 95kPa
- Contents are listed on requisition or sample submission form or otherwise listed outside of secondary packaging
- Secondary packaging is secure inside outer packaging, with extra cushioning if necessary
- Paperwork is protected from condensation from chemical ice packs, if used
- If dry ice is used, proper dry ice shipping protocols have been referenced and followed
- Outer packaging is rigid and in good condition
- UN3373 hazard diamond and “Biological Substance, Category B” labels are present on outer package and not touching the edge of any side
- Responsible person name, address, phone number are listed on outer packaging
- Consignee and Shipper name, address, and telephone number are listed on outer packaging
- All old or non-applicable labels are removed or covered
Dry Ice
Dry Ice is considered hazardous material. Specimens shipped frozen on dry ice must be packaged in dry-ice ready containers and labeled according to IATA and DOT requirements. Dry ice may be used in an insulated container that is sealed loosely to permit escape of sublimated carbon dioxide gas. Accumulated gaseous CO2 trapped in an airtight container can create high pressure and an explosive hazard.
Resources for Shipping Category A, B, and Exempt Specimens
Pipeline and Hazardous Materials Safety Administration (PHMSA)
Federal Express Dangerous Goods/Hazardous Materials Hotline
IATA Dangerous Goods Regulations
Shipping Select Agents
Suspect select agents sent for confirmatory testing by the Bioterrorism and Emergency Response Team should be shipped as “UN 2814 Infectious Substance, Affecting Humans (Suspected Category A Infectious Substance)”. Never include the suspected identity of the organism being transported on outer packaging or shipping papers. Information pertaining to suspected identity and testing should only be present on the test requisition which shall be placed between the secondary and outer packaging and protected from condensation due to ice packs, if used. All submissions of suspect select agents must be pre-approved by the appropriate Local Health Jurisdiction and the Washington State Office of Disease Control and Health Statistics Epidemiology Department. Confirmed select agents may not be shipped to WAPHL.
Washington State Department of Health
Washington State Public Health Laboratory
1610 NE 150th Street
Shoreline, WA 98155
Phone: 206.418.5400
Shipping Forms and Instructions
Containers
Corporate One West
1195 Washington, Pike
Bridgeville, PA 15017-2854
Phone: 800-245-2283
FAX: 412-257-3001
800 Industrial park Road
P.O. Box 2707
Carbondale, IL 62902
Phone: 800-824-0817
FAX: 618-529-2234
3 Milltown court
P.O. Box 1690
Union, NJ 07083
Phone: 800-577-7624
FAX: 908-687-5157
7416 North Broadway
Extension, Suite E
Oklahoma City, OK 73116
Phone: 800-866-7172
FAX: 405-848-7701
101, 17872 - 106 Avenue
Edmonton, Alberta, Canada
T5S 1V4
Phone: 800-841-7484
FAX: 403-486-0235
Self-Seal Container Corporation
401 E. Fourth Street
Bridgeport, PA 19405
Phone: 800-334-1428
FAX: 610-275-4430
306-A White Street South
Wake Forest, NC 27587
Phone: 800-266-0652
FAX: 919-554-9055
Sonoco Absorbent Technologies (Medicor)
31 Industrial Park Road
Wapato, WA 98951
Phone: 843-383-7712
FAX: 843-383-7222
Source Packaging of New England, Inc.
405 F Kilvert Street
Warwick, RI 02866
Phone: 800-200-0366
FAX: 401-738-7762
Shipping Labels
5724 N Pulaski Rd
Chicago, IL 60646
Phone: 1-800-621-5808
Fax: 1-800-723-4327
5361 Alexander Street,
Los Angeles, CA 90040
Phone: 1-800-745-8800
